I reviewed the De Novo Classification request for the “Hearing Aid Feature” (HAF) which refers to the software within the AirPods Pro 2 (hardware) to allow them to function as OTC hearing aids. It is described as “a software-only mobile medical application that is intended to be used with compatible wearable electronic products”. The indications for use follow the FDA criteria for OTC hearing aids to specify use for people 18 years and older with perceived mild to moderate hearing impairment.
Below is a screenshot of the Instructions for use. I am pleasantly surprised to see language directing the end user to seek additional help from a hearing healthcare professional if they have continuing hearing concerns or limitations to the Hearing Aid Feature functionality.

Use and Set-Up of HAF
To use the HAF, a hearing exam needs to be input into the software. The HAF allows for an audiogram to be obtained either from the HAF software “Hearing Test Feature” or from a hearing healthcare professional. Apples “Hearing Test Feature” (HTF) was validated via a data set of 202 participants . The results revealed there were similar pure-tone averages for audiograms derived from the HTF and professionally obtained audiograms for each participant. The HAF requires an audiogram that includes all standard octave frequencies (250Hz, 500Hz, 1000Hz, 2000Hz, 4000Hz, and 8000Hz). Once the hearing exam is entered into the software, the process is self-guided and provides step-by-step instructions and warnings for use as the end user progresses through set up.
Once the user enters their Audiogram, reads the warnings, and verifies their age, the software will adjust the amplification to the users audiogram based on “Apple’s proprietary fitting formula”. After initial fitting, users can fine tune the sound (amplification, tone, and balance) from controls on their iOS device. The HAF settings are stored in the “HAF Firmware Module” which takes advantage of the microphone, speakers, amplifier and audio processing software within the AirPod Pro.
Once a user sets up their AirPods with the HAF, the settings are stored in the HAF Firmware Module and are available when the AirPods Pro are not connected to the device.
Clinical Verification
The purpose of the clinical verification study was to assess the performance of the HAF against the National Acoustic Laboratories NAL-NL2 fitting formula. NAL-NL2 is a standard fitting strategy that hearing care professionals use to adjust prescription hearing aids. 118 participants were included in the study. Participants included were not hearing aid users. Study participants were divided into “self-fit” or “professionally-fit” groups. Both groups utilized the AirPod Pro 2 as the hardware and both groups had professionally derived audiograms used as the basis for the fitting. The outcome measure used was the International Outcome Inventory for Hearing aids (IOI-HA).
Users were assessed over a 31 day span . Users in the professionally-fit group had their AirPods fit by an Audiologist at the initial visit. Users in the self-fit group were instructed to follow the set-up instructions of the HAF on their iOS device. Once the AirPods were fit in each group, end users were able to have their settings fine tuned either at a follow-up home visit (professionally-fit group) or through the HAF user controls on their iOS device.
Study participants were required to use their devices at least 30 minutes per day. Results of the IOI-HA outcome measure revealed that the self-fit users achieved the same perceived benefit as the professionally-fit group. In addition to IOI-HA outcome measures, objective measures for Speech in Noise testing (QuickSIN) and Real Ear Measures (REM) were collected. There was no significant difference in speech intelligibility or amount of gain provided in either the self-fit or professionally-fit group.
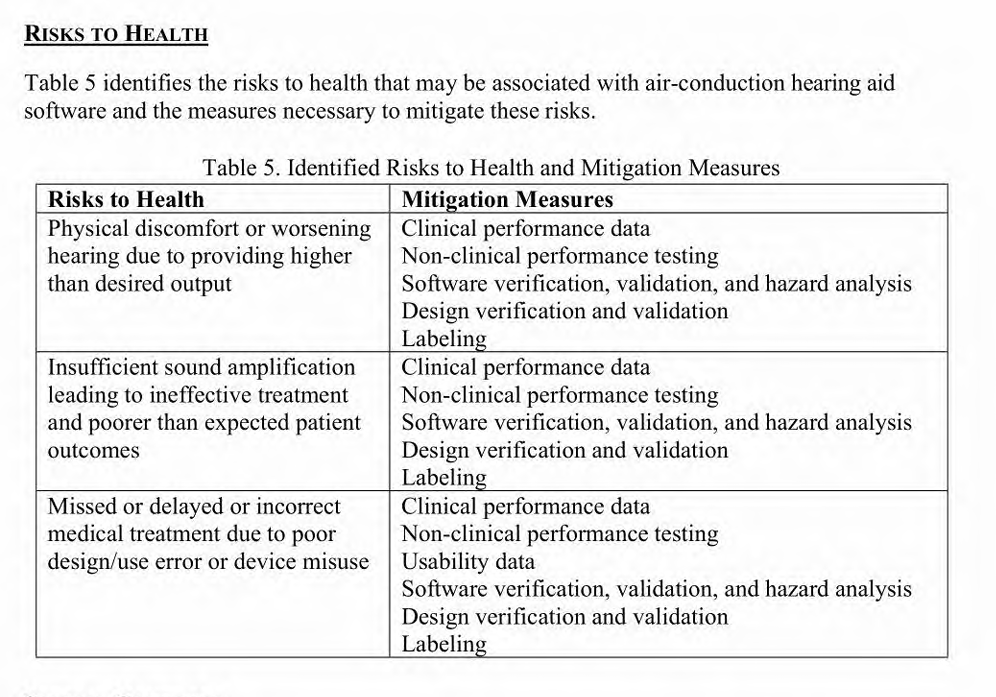
Risks to Health
Included within the De Novo request is information regarding the risks to health that maybe associated with the device. Below you will see the identified risks and their mitigation measures. It was determined that there was a low-to-moderate risk profile for the HAF and that the benefits outweigh the risk of use of the HAF.
In summary, HAF in AirPods Pro 2 offers promising results, making hearing assistance more accessible while advising professional consultation when needed. This innovative feature could revolutionize the way individuals manage their hearing health, offering a convenient and effective solution right from their phones. By incorporating professional guidance and validation, Apple ensures that users are not only empowered by cutting edge technology but also supported in making informed decision about their hearing care. As we move forward, such advancements underscore the importance of blending technology with professional expertise to enhance quality of life for those with hearing impairments.
